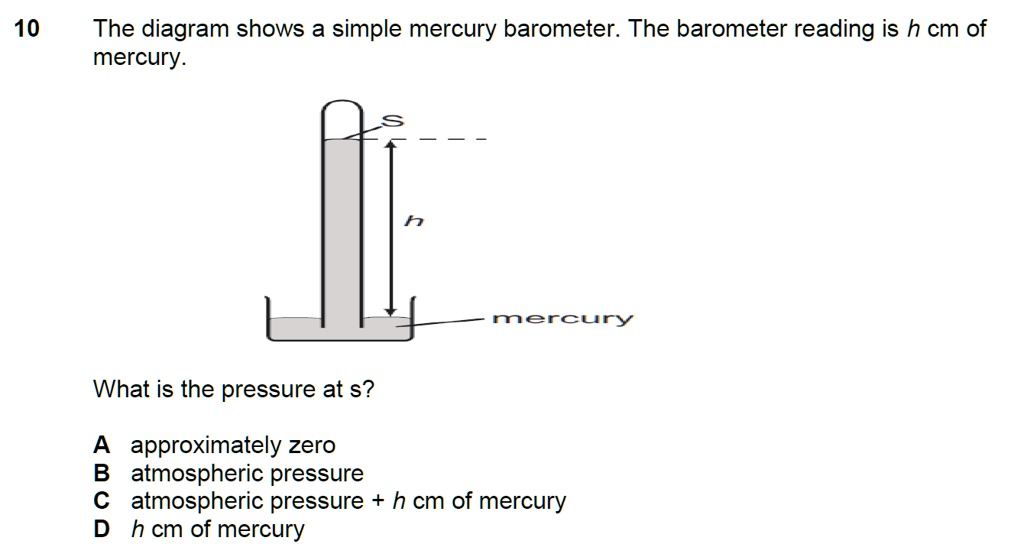
What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm . Gas pressure is a gauge of the number and force of collisions between gas particles and the. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. the ideal gas law describes the property of a hypothetical ideal gas. a sample of the gas at a pressure of 727 mmhg and a temperature of 18°c weighs 0.289 g in a flask with a volume of 157.0 ml. Barometer is an instrument that is used to measure atmospheric pressure. the height of the mercury in a barometer is directly proportional to the pressure on the mercury's surface. Pv=nrt in which p=pressure, v=volume, n=moles of. air pressure is measured with a barometer. enter the values of volume (v), number of moles (n) and absolute temperature (t) below which you want to find the pressure of an.
from www.numerade.com
a sample of the gas at a pressure of 727 mmhg and a temperature of 18°c weighs 0.289 g in a flask with a volume of 157.0 ml. enter the values of volume (v), number of moles (n) and absolute temperature (t) below which you want to find the pressure of an. the ideal gas law describes the property of a hypothetical ideal gas. air pressure is measured with a barometer. Barometer is an instrument that is used to measure atmospheric pressure. Pv=nrt in which p=pressure, v=volume, n=moles of. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. the height of the mercury in a barometer is directly proportional to the pressure on the mercury's surface. Gas pressure is a gauge of the number and force of collisions between gas particles and the.
SOLVED The diagram shows a simple mercury barometer. The barometer
What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Barometer is an instrument that is used to measure atmospheric pressure. enter the values of volume (v), number of moles (n) and absolute temperature (t) below which you want to find the pressure of an. air pressure is measured with a barometer. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. Gas pressure is a gauge of the number and force of collisions between gas particles and the. the ideal gas law describes the property of a hypothetical ideal gas. a sample of the gas at a pressure of 727 mmhg and a temperature of 18°c weighs 0.289 g in a flask with a volume of 157.0 ml. Pv=nrt in which p=pressure, v=volume, n=moles of. Barometer is an instrument that is used to measure atmospheric pressure. the height of the mercury in a barometer is directly proportional to the pressure on the mercury's surface.
From cecuannx.blob.core.windows.net
What Are Barometric Pressure Ranges at Douglas Hall blog What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm the ideal gas law describes the property of a hypothetical ideal gas. Barometer is an instrument that is used to measure atmospheric pressure. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. a sample of the gas at a pressure of 727 mmhg and a temperature of 18°c weighs. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.numerade.com
SOLVED The diagram shows a simple mercury barometer. The barometer What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. Gas pressure is a gauge of the number and force of collisions between gas particles and the. the height of the mercury in a barometer is directly proportional to the pressure on the mercury's surface. enter the values of volume. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From mavink.com
Barometric Pressure Chart What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm the height of the mercury in a barometer is directly proportional to the pressure on the mercury's surface. Pv=nrt in which p=pressure, v=volume, n=moles of. Barometer is an instrument that is used to measure atmospheric pressure. Gas pressure is a gauge of the number and force of collisions between gas particles and the. air pressure is measured with. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.youtube.com
pressure, conversion units into defferent units,atm,bar,torr,psi,Pascal What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Gas pressure is a gauge of the number and force of collisions between gas particles and the. the ideal gas law describes the property of a hypothetical ideal gas. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. a sample of the gas at a pressure of 727 mmhg. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From djcwmkdceco.blob.core.windows.net
How To Make A Barometer To Measure Air Pressure at Stephen Keese blog What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm the ideal gas law describes the property of a hypothetical ideal gas. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. Barometer is an instrument that is used to measure atmospheric pressure. a sample of the gas at a pressure of 727 mmhg and a temperature of 18°c weighs. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.youtube.com
Barometers measure Atmospheric Pressure YouTube What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm air pressure is measured with a barometer. the height of the mercury in a barometer is directly proportional to the pressure on the mercury's surface. a sample of the gas at a pressure of 727 mmhg and a temperature of 18°c weighs 0.289 g in a flask with a volume of 157.0 ml. Gas pressure is a. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From ceedliji.blob.core.windows.net
What Is Barometric Pressure And How Does It Work at Charles Brennan blog What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Gas pressure is a gauge of the number and force of collisions between gas particles and the. Barometer is an instrument that is used to measure atmospheric pressure. the ideal gas law describes the property of a hypothetical ideal gas. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. . What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From sciencing.com
How to Understand Barometric Pressure Readings Sciencing What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm a sample of the gas at a pressure of 727 mmhg and a temperature of 18°c weighs 0.289 g in a flask with a volume of 157.0 ml. air pressure is measured with a barometer. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. enter the values of. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.chegg.com
Solved Given a barometric pressure of 749.5 mmHg , What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Barometer is an instrument that is used to measure atmospheric pressure. Pv=nrt in which p=pressure, v=volume, n=moles of. air pressure is measured with a barometer. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. the ideal gas law describes the property of a hypothetical ideal gas. a sample. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From calculatorport.com
mmHg to atm converter Easy to convert CalculatorPort What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm the ideal gas law describes the property of a hypothetical ideal gas. enter the values of volume (v), number of moles (n) and absolute temperature (t) below which you want to find the pressure of an. Pv=nrt in which p=pressure, v=volume, n=moles of. Barometer is an instrument that is used to measure atmospheric pressure. The height of the. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From quizlet.com
2.18 What would be the barometric pressure reading, in mmHg Quizlet What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. Gas pressure is a gauge of the number and force of collisions between gas particles and the. Barometer is an instrument that is used to measure atmospheric pressure. the height of the mercury in a barometer is directly proportional to the. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.wikihow.com
How to Calculate Barometric Pressure 6 Steps (with Pictures) What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Pv=nrt in which p=pressure, v=volume, n=moles of. Barometer is an instrument that is used to measure atmospheric pressure. enter the values of volume (v), number of moles (n) and absolute temperature (t) below which you want to find the pressure of an. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.dracal.com
How to measure atmospheric pressure? Dracal Technologies What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm enter the values of volume (v), number of moles (n) and absolute temperature (t) below which you want to find the pressure of an. Barometer is an instrument that is used to measure atmospheric pressure. air pressure is measured with a barometer. a sample of the gas at a pressure of 727 mmhg and a temperature of. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From quizconsectary.z21.web.core.windows.net
Pascals To Atm Conversion What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Gas pressure is a gauge of the number and force of collisions between gas particles and the. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. air pressure is measured with a barometer. the height of the mercury in a barometer is directly proportional to the pressure on the. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.slideserve.com
PPT Recording Measurements Makeup Lab PowerPoint Presentation, free What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm the height of the mercury in a barometer is directly proportional to the pressure on the mercury's surface. Barometer is an instrument that is used to measure atmospheric pressure. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. enter the values of volume (v), number of moles (n) and. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.numerade.com
SOLVED Part A The barometer shown here (Figure 1) measures atmospheric What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Barometer is an instrument that is used to measure atmospheric pressure. enter the values of volume (v), number of moles (n) and absolute temperature (t) below which you want to find the pressure of an. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. the height of the mercury. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From ceoofasc.blob.core.windows.net
How To Calibrate Barometric Pressure at Andrew Chandler blog What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm Barometer is an instrument that is used to measure atmospheric pressure. the ideal gas law describes the property of a hypothetical ideal gas. The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. Gas pressure is a gauge of the number and force of collisions between gas particles and the. . What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.
From www.wikihow.com
How to Calculate Barometric Pressure 6 Steps (with Pictures) What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm The height of the mercury in a barometer is directly proportional to the pressure on the mercury’s surface. Barometer is an instrument that is used to measure atmospheric pressure. air pressure is measured with a barometer. the ideal gas law describes the property of a hypothetical ideal gas. Pv=nrt in which p=pressure, v=volume, n=moles of. a sample. What Is The Barometer Reading In Mmhg At A Pressure Of 0.50 Atm.